The Fundamentals – HCOOH, CH₂ , H₂O
Chemical formulas such as HCOOH formic acid, the simplest carboxylic acid, H₂O (Water), and CH₂ which translates to Methylene group (a common fragment in organic synthesis) might be cryptic, but they fundamentally compose and govern our daily activities.
In water’s dominancy as a polar solvent, formic acid stretches its utility across both natural and industrial realms. Methylene groups may not be often isolated, yet their ability to extend hydrocarbon chains proves output dominance in polymers and fuels, quietly molding biochemicals.
Molecule Visualization: Chemical Structures
HCOOH has a planar structure with a carboxylic acid -COOH group; there is a double bond with one oxygen, and a bond with a hydroxyl group (OH) which is single. The hydrogen attached to the carboxyl group makes it acidic and donates protons easily in water. Accepting H+ ions from aqueous solutions.
A methylene group is represented by CH2, or a single carbon atom with two hydrogen atoms attached to it. In most organic compounds, it is present as part of a long hydrocarbon chain where it connects to other carbon atoms. While it is not a building block that is usually found on its own, it is still a key part of molecular structures.
The water molecule, H2O, is V or bent shaped due to the arrangement of bond pairs and lone pairs. This gives the molecule angularity which paired with the electronegative nature of oxygen, accounts for the high polarity of water. Its ability to easily form hydrogen bonds makes it a key player as a universal solvent and in thermal stability.
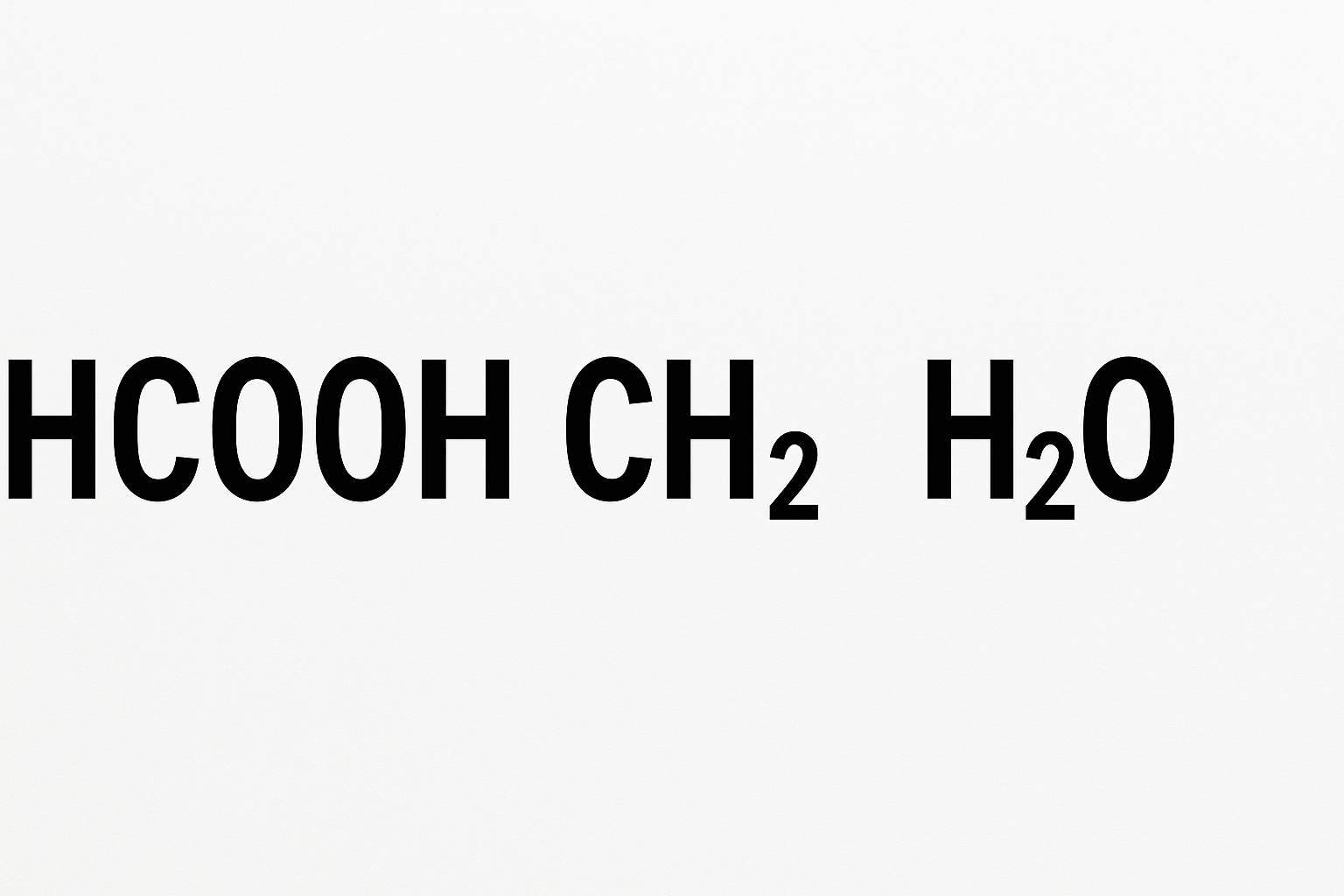
Sources and Natural Occurrence
Formic acid (HCOOH) is present in the venom of ants and the sting of nettles. Its name comes from the Latin term formica which means ant. It is used by some insects as a defense mechanism and also as a byproduct of certain microbial fermentation processes.
While not found in nature as a standalone molecule, CH₂ is a common part of organic compounds which include fatty acids, amino acids, and several hydrocarbons.
Water (H₂O) is the most abundant liquid in nature. It exists over a geographical expanse of 71% of Earth’s surface and goes through an unending cycle of evaporation, condensation, and precipitation. Water is also produced during many chemical reactions such as combustion and respiration.
Industrial Applications and Daily Utility
Formic acid is used in textile formulating, leather tanning, as well as a preservative in animal feed. In rubber production, she acts as a coagulant, and as a component in household descalers. In scientific laboratories, she is appreciated for reduction reactions.
As parts of alkanes and polymers, CH₂ groups are the building blocks of contemporary industry and invention. Flexibility in the form of –CH₂– units, polyethylene plastic is utilized in grocery bags, prosthetics, and is essential in synthetic fuels and lubricants.
No one can match water’s usefulness. It is a coolant, solvent, transporter, reactant, habitat, washes, cooks, cools, and hydrates. In industries, it is integral in cooling towers, reactors, cleaning systems, and energy generation.
Biological Importance and Interrelationships
Formic acid is important for certain metabolic pathways and cellular respiration processes, especially in anaerobic microorganisms. In humans, format (formic acid’s ionized version) is toxic in high concentrations but a byproduct of metabolism in smaller quantities.
The CH₂ group is part of amino acids such as glycine and alanine, having significance on the structural level of proteins and enzymes. Furthermore, it is found in the backbones of DNA, which helps maintain and control its vital functions in heredity and expression.
Life hinges on water. Every biochemical reaction occurs in an aqueous medium. Water regulates cellular temperature, nutrient transport, and biochemical processes. In addition, the hydrogen bonds of water aid in folding proteins, pairing of DNA strands, and maintaining cellular structures.
Environmental and Ecological Issues
Cloud and soil chemistry can be affected by formic acid which, though weak in strength, contributes to atmospheric acidity. Biomass burning and vehicle emissions both release formic acid.
Hydrocarbons are majorly composed of compounds containing CH₂. These molecules, more specifically, petroleum products, can contaminate ecosystems and introduce persistent organic pollutants into the food chain further endangering the environment.
The rising global and regional inequalities in water scarcity and pollution is one of the world’s most critical issues. This occurs both due to industrial activities discharge and plastic pollution overuse. Conserving water is essential for humans, and solving this issue has a lot of potential benefits to humankind.
Future Perspectives in Research and Innovation
Considering alternative energy sources, formic acid is examined as a possible hydrogen carrier in fuel cells. Formic acid’s capability of decomposing to hydrogen and carbon dioxide under mild conditions makes it beneficial for sustainable power technologies.
The CH₂ groups are crucial in designing new polymers and carbon nano structures. There is an emphasis on chains with methylene linkages for biodegradable plastics as they are easier to break down than long lasting hydrocarbons.
Water is the foundational component in future sustainability schemes. To provide access to clean water, desalination, water harvesting, and nano-filtration technologies are being developed. Water also plays a critical role in climate models as the oceans and ice caps set the weather and temperatures around the globe.
Conclusion
While HCOOH, CH₂, and H₂O appear as mere combinations of atoms, they are essential to life, industry, and innovation. Their actions are chemical processes shaped by their structures, and their existence—or manipulation—affords orchestrated processes that are foundational to life. These molecules are invisible to us, yet permeate every corner of our existence, from the flow of water, the sting of an ant, synthetic polymers, and even the food we consume.







